Just ahead of Halloween, Duke biomedical engineering researchers have developed an artificial protein, known as the “Frankenstein protein,” that can help repair damaged body parts.
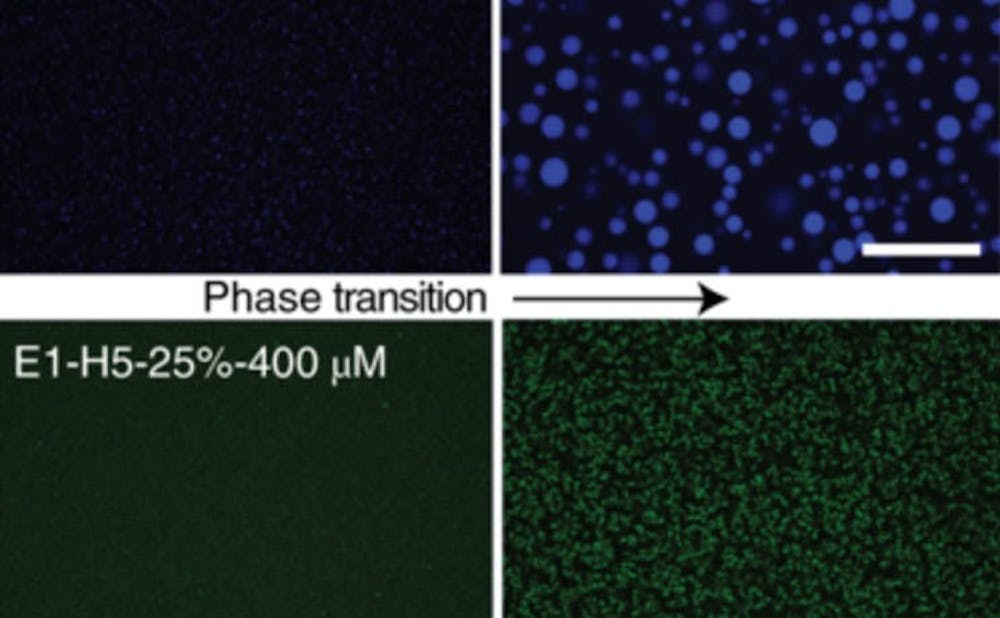
The research team developed artificially generated elastin, an extracellular, elastic protein that acts like a fluid at lower temperatures and forms an aggregate at higher ones, said Stefan Roberts, first author of the paper and Ph.D. student at Duke.
A large component of connective tissue, elastin allows tissues to return to their original form after being disturbed, making it a natural material to use in tissue engineering and regenerative medicine. Researchers in the collaborative study between Duke and the Pappu Lab at Washington University in Saint Louis generated the protein by decomposing animal tissues and synthesizing them with artificial material, making it a "Frankenstein protein," Roberts explained.
Elastin has been studied for decades, but researchers found limited success in translating designed elastin-like proteins into ones that could be effective in tissue regeneration, Roberts explained.
"This is finally a positive step in that direction,” Roberts said.
Rohit Pappu, Edwin H. Murty professor of engineering and principal investigator of the Pappu Lab at Washington University in Saint Louis, described elastin as having both “rigid structures” as well as softer ones dubbed “spaghetti.” Researchers at the Pappu Lab had been studying the spaghetti-like proteins before their collaboration with the team at Duke.
The researchers have manipulated elastin so that when they inject and integrate the protein mechanically into the body, it would still maintain rigidity and create a strong scaffolding. After it serves its purposes, the protein will react to body temperature and return to a liquid state.
With these features, the new protein could be used for diabetic and surface ulcers, as a component between flaps of skin grafts, and other needs.
Which rigid segments are added to the protein polymer determine at what temperature it will form a solid, and when it will fall apart. The rigid structures also determine how much memory the protein will have, Pappu said.
“For a skin graft, you might want material that lives in tissue for a year and falls apart," he added. "So we will change the ratio of ordered to disordered regions and change the hysteresis loop.”
Roberts said he and his colleagues observed elastin's unique property when they were investigating it for other purposes.
“Nature has spent millennia designing materials with specific biological functions," Roberts said. "We are very interested in the idea that we could take those out and redesign them for natural purposes.”
Currently, Roberts plan to market this new protein, as there is no other injectable material based in elastin that can be used in such a wide variety of applications. Now as a biomedical engineering entrepreneurial fellow, Roberts will spend the next year to spread its use by collaborating with additional researchers and clinicians at Duke.
Pappu's team, on the other hand, will continue to study the protein and its molecular memory in the context of other diseases.
“We know what nature does. You can mimic nature, or you can say that we don't need to be constrained by it,” Pappu said.
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.

Maria Morrison was a digital strategy director for The Chronicle's 117th volume. She was previously managing editor for Volume 116.