A recent analysis from the University of Oxford suggests that Duke is among the worst universities in the United States for reporting clinical trials.
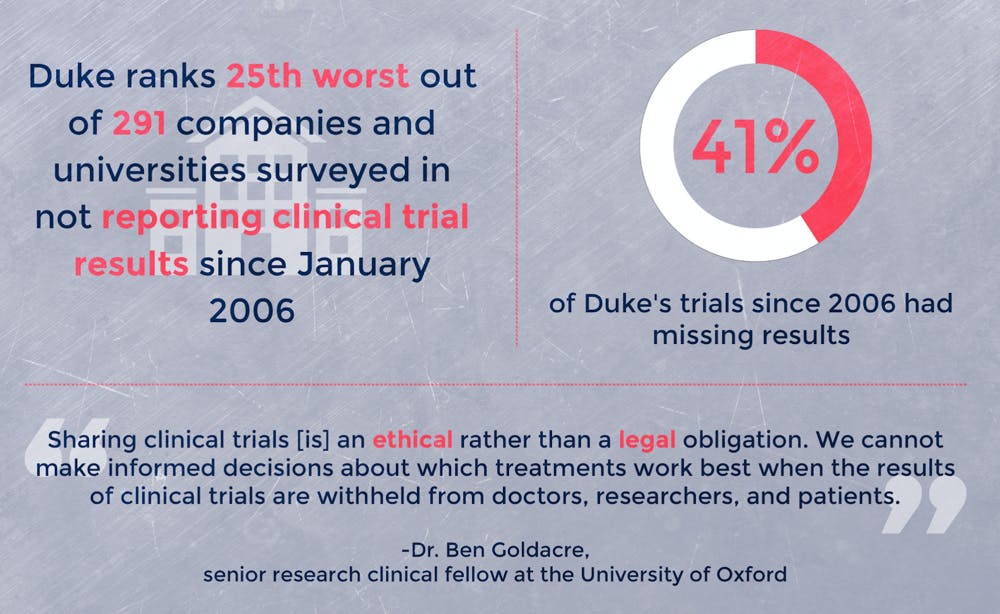
The report compiled clinical trial statistics through ClinicalTrials.gov, a federal database of all government-funded and many privately-funded studies. Using the data, the investigators found that several companies and universities had neglected to report or publish findings resulting from their clinical trials. This includes Duke, which is missing results for 41.9 percent of all trials since 2006, according to the analysis.
Although only certain types of clinical trials are legally required to be published, these findings nonetheless have ethical consequences, argued Dr. Ben Goldacre, a senior research clinical fellow at the University of Oxford and leader of the study.
“Sharing clinical trials [is] an ethical rather than a legal obligation,” he wrote in an email. “We cannot make informed decisions about which treatments work best when the results of clinical trials are withheld from doctors, researchers and patients.”
The most recent results were published in October 2016 as part of the University of Oxford’s TrialsTracker website in partnership with the AllTrials initiative. According to TrialsTracker, Duke ranks 25th worst out of 291 companies and universities surveyed in not reporting clinical trial results since January 2006. Out of universities in the United States, Duke is better than some peer institutions such as Stanford University and the University of Pennsylvania.
But just because some trials are not being reported does not mean Duke has not complied with legal requirements, noted Denis Snyder, associate dean for clinical research. She added that Duke itself has been in compliance with federal guidelines for Applicable Clinical Trials.
According to Food and Drug Administration regulations, only Applicable Clinical Trials—specific types of trials related to biologics, drugs and medical device—must be reported within a year of study completion.
"The [TrialsTracker article] doesn’t differentiate between all clinical trials and ACTs," she told The Chronicle. "Published results are required only for ACTs and beginning in 2007. The article addresses trials from 2006. Lack of differentiation between all trials and ACTs changes the data as reported in the article. Duke is at 100 percent compliance with the law regarding requirements to publish clinical trial results.”
A manual review by The Chronicle starting from 2007 found that all ACTs for which Duke was the primary sponsor were in compliance with FDA guidelines. There were eight examples, however, of trials that could be considered ACTs according to FDA guidelines that did not report study findings, but Duke was not the primary sponsor on these. The remaining trials that went unreported were behavioral or observational in nature or otherwise exempt from legal reporting requirements.
Goldacre noted that these ACT regulations nationwide “have been widely ignored, with compliance rates of only one in five.” A study by Duke investigators in 2015 found that only 13.4 percent of all ACTs were reported in line with with FDA guidelines within one year of study completion.
Companies and academic institutions are still encouraged to report all trials even if not required to do so, explained Trudo Lemmens, Scholl Chair in health law and policy at the University of Toronto Faculty of Law.
“Correct representations of findings is a core ethical requirement associated with respect for those who participated in research (i.e. you don't allow people to be used as research subjects in a pure marketing scheme) and is also required to avoid harm to future patient and to avoid unnecessary costs to the health care system,” he wrote.
Some researchers noted that some unpublished trials listed on ClinicalTrials.gov might still be in the works.
In 2013, Dr. Momen Wahidi, associate professor of medicine, completed a study on the dynamics and efficiency of lung drainage using a catheter device. Although no results from the study are currently listed on ClinicalTrials.gov, Wahidi noted in an email that he and his colleagues planned to update the database soon.
"The [catheter trial] actually just got accepted for publication and will be published in a few months,” he said. “It just takes a long time to conduct trials, analyze data then get it accepted into a journal.”
Other physicians and faculty at Duke who were listed as the lead investigators for clinical trials with unreported results could not be reached for comment.
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.