The Duke Human Vaccine Institute has renewed its seven-year contract with the National Institute of Health to perform quality control tests for HIV research.
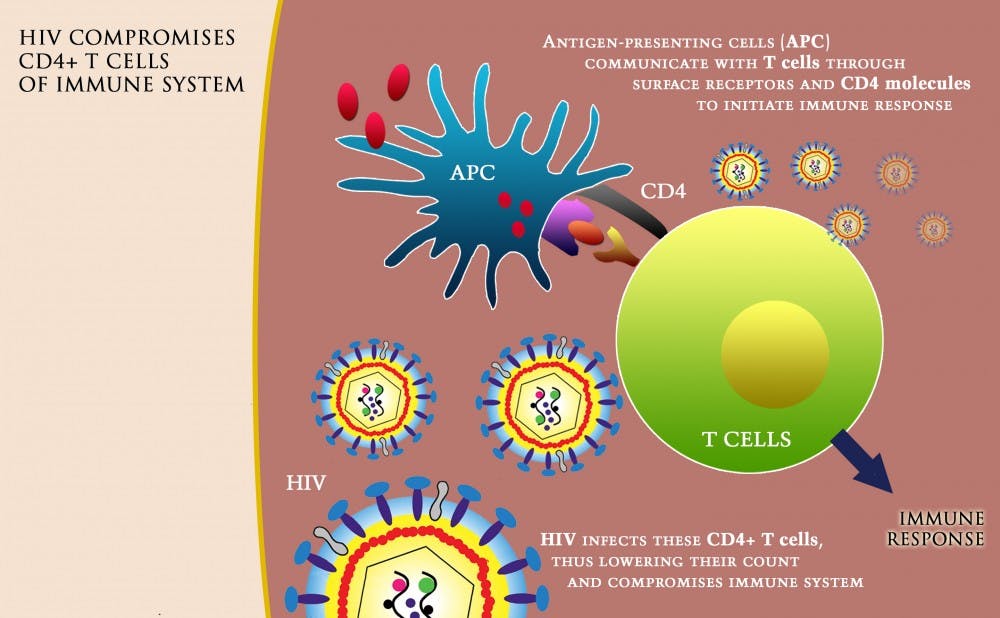
The contract enables Thomas Denny, chief operating officer of DHVI, and his laboratory team to evaluate both domestic and international assays of CD4 cells—a type of T-lymphocyte infected by the HIV virus. The count of these cells is used to determine the strength of a patient's immune system. Denny, who serves as principal investigator of the project, has held this contract with the NIH since 1999. The contract puts him in charge of 80 labs throughout the US and an additional 70 labs internationally.

“It puts us in the spotlight,” Denny said. “There’s only one of these programs funded by the NIH, so it’s one of a kind.”
Because Denny is responsible for both domestic and international labs, his team travels all over the world to perform these quality assessments. The lab inspections his team conducts involve checking the supplies and necessary reagents as well as teaching and training other researchers. He added that because all labs should be performing the same test, the process should not be affected by the use of different equipment brands.
“You’re enrolling patients for therapies all around the world, and you’re eventually going to bring all that data together in one database—so you want to make sure that tests that are done in South Africa are the same as the ones done in New York or Paris,” Denny said.
This contract could be worth up to $13.9 million over the course of seven years—which includes equipment costs, travel fares and the salaries of team members, Denny said.
Denny was initially awarded the contract at New Jersey Medical School, and had it transferred when he came to Duke in 2006.
“Having Tom Denny’s quality assessment infrastructure here at Duke provides unique infrastructure for both the Center for HIV/AIDS Vaccine Immunology work we do on the AIDS vaccine and for all of the Duke Human Vaccine Institute’s work on other emerging infections such as influenza and TB,” said Director of DHVI Barton Haynes.
The contract has enabled Denny and his lab to generate and gain access to a large amount of data. He added that having certified labs allows the Institute to be more competitive when applying to grants, which provide for future research.
“It’s going to help us to be a leader in diagnostics and set standards for assays that are used in clinical trials,” Denny said. “Hopefully, it will enable us to continue to strengthen our research and academic mission at Duke.”
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.