A new study by the Sullivan Lab suggests that a major breakthrough in better understanding cancer might be hiding in plain sight.
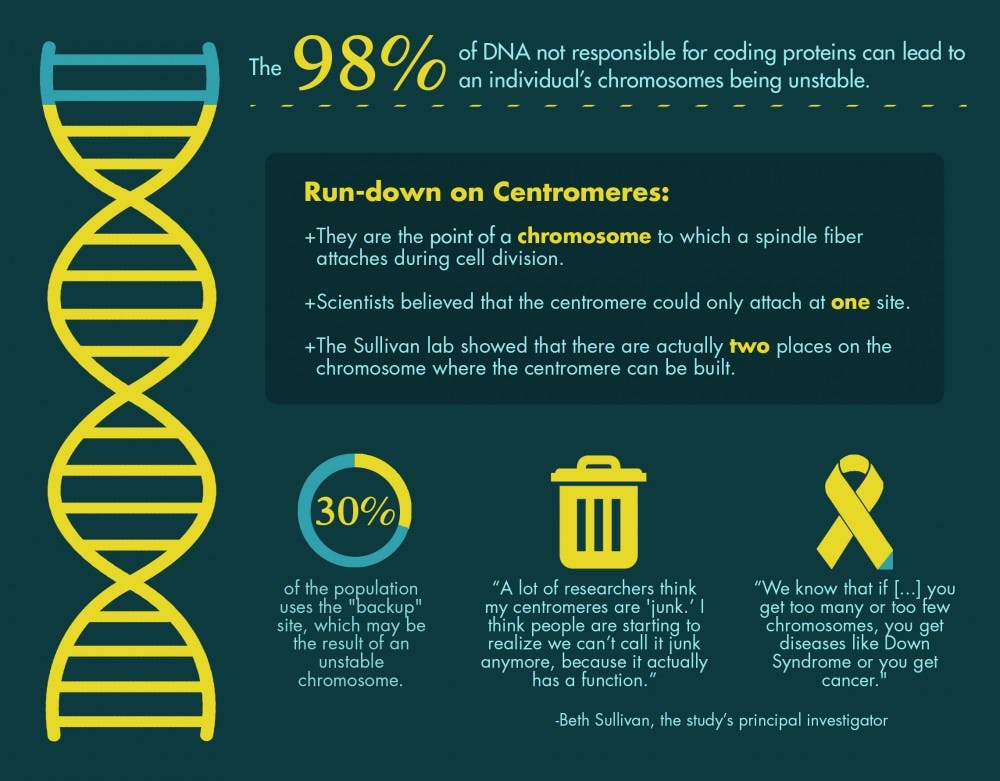
Using a new technique, researchers found that variations in sequences of satellite DNA—the 98 percent of DNA not responsible for coding proteins—can lead to an individual’s chromosomes being unstable. This puts some at much higher risk of developing cancer.
“My basic reason for doing what I do is to understand how chromosomes are precisely inherited every cell cycle, every cell division," said Beth Sullivan, the study’s principal investigator and an associate professor of molecular genetics and microbiology. "We know that if this process is not carried out precisely, if you get too many or too few chromosomes, you get diseases like Down Syndrome or you get cancer."
Sullivan’s study was interested in the 98 percent of DNA—commonly referred to as junk DNA—that does not code for proteins in the body. These segments are often repetitive and lack the amount of variation found in protein-coding DNA.
Specifically, she studies centromeres, the point of a chromosome to which a spindle fiber attaches during cell division.
“A lot of researchers think my centromeres are 'junk,'" Sullivan said. "I think people are starting to realize we can’t call it junk anymore, because it actually has a function. Now we’re realizing it can actually have some serious functional consequences."
Sullivan, who also serves as director of the Genetics and Genomics FOCUS cluster, uses the analogy of a bus when describing centromeres and their functions to her students. If the chromosome is a bus, the centromere is the bus driver—it is the component that drives the movement of the chromosome. Most researchers study impairments of the bus, but Sullivan believes the bus driver is just as important.
The Sullivan Lab has previously made other discoveries in the field. Scientists long believed that the centromere could only attach at one site until several years ago when the Sullivan lab showed that there are actually two places on the chromosome where the centromere can be built. They found that 30 percent of the population used the "backup" site, which may be the result of an unstable chromosome.
“We wanted to know why there was an alternative, what was the need? What are the genetic conditions that would cause you to use a backup site?" Sullivan said. "Our hypothesis was that you use the backup site if there was genomic variation at the primary site which causes it to be less desirable.”
o stretch out these tiny, condensed regions, the team built a small platform and secured to it to three microscope slides and cover slips.
“What this did was allow me to be able to put my cells on the slide in the middle and take another slip and move down along the slide without actually touching or breaking the DNA that was being stretched out. We were able to stretch out the array and then use [fluorescence in situ hybridization] to tag specific parts of interest,” said Molly Kuo, Trinity ’15 and a co-author of the study. “It was really cool. I felt like I got to build something new, which was different from my everyday work.”
Sullivan is currently seeking funding to research the 30 percent of the population with unstable DNA.
“I’m on a campaign to get people to realize that these regions of the genome perform very essential functions, that they have variation just like genes do and that we really need to be studying them too," Sullivan said. "It’s like my personal crusade."
In the future, Sullivan hopes to unite this study with the overarching goal of her lab—building a fully functional artificial chromosome.
“The goal of the artificial chromosome is to put any gene on it you want," Sullivan said. "You can use it for gene therapy, you can correct certain diseases, you can correct a blood disorder by adding on a good factor in a cell that didn't previously have it. Our foundational goal is to build the best centromere possible, and then add genes to it that can be used to correct disease.”
Get The Chronicle straight to your inbox
Signup for our weekly newsletter. Cancel at any time.